Cosmetic Import Registration

What is Cosmetic Import Registration?
“Cosmetic” means any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and includes any article intended for use as a component of cosmetic. The demand of cosmetics has been increasing globally due to the rising prevalence of skin and hair disorders and increasing awareness of dermatological treatments. However, it puts a significant pressure on companies to keep up with global regulations. MAC can help you to combat the regulatory challenges and therefore reduce time and risks for the industry people.
Who regulates Cosmetics in India?
The Central Drugs Standard Control Organisation (CDSCO) under Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India is the National Regulatory Authority (NRA) of India.

What is Import Registration certificate?
Import registration certificate means a certificate issued under rule 13 by the Central Licensing Authority for registration of cosmetics manufactured for import into and use in India. An application for registration of a cosmetic product intended to be imported into India shall be made through the online portal of the Central Government in Form COS-1. On being satisfied the Central Licensing Authority grants import registration certificate in Form COS-2, formerly known as Form-43.
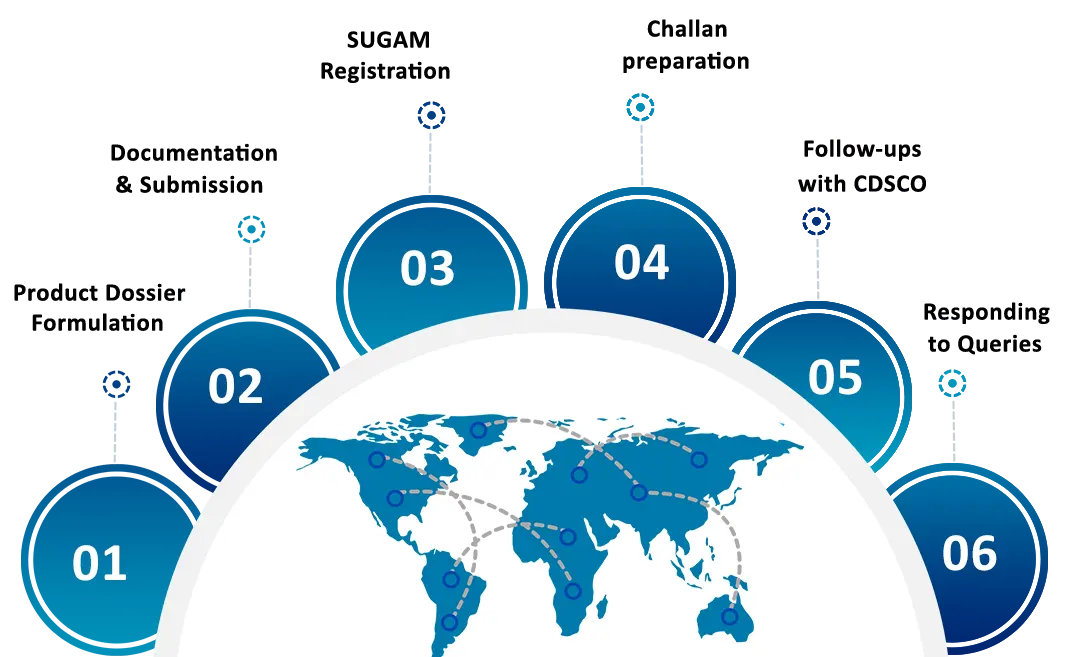
How we help your business?
Move Ahead Consultancy provides assistance in filing application of Import Registration (COS II) Certificate of cosmetic products for Import into the country as per the requirements of Drugs and Cosmetics Act 1940 and Cosmetic Rules made there under in the following ways: